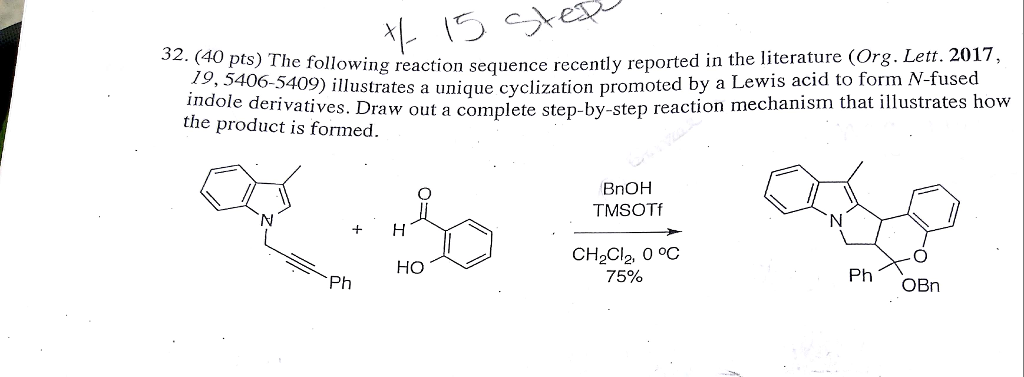
Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3. - Abstract - Europe PMC
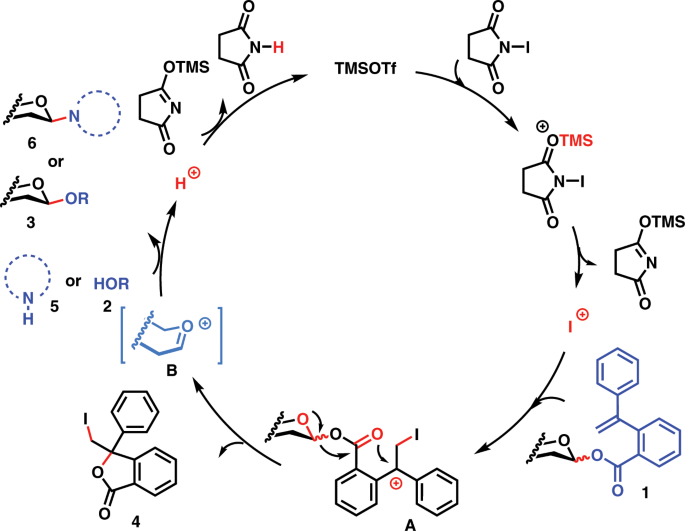
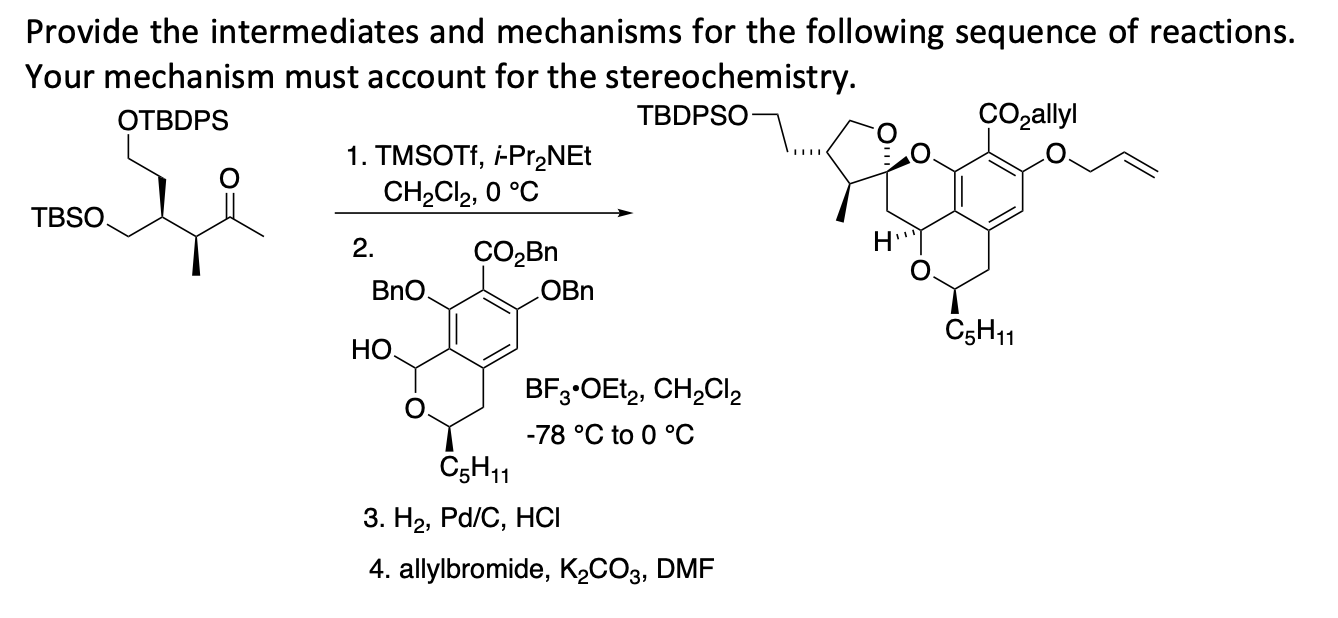
Glycosyl ortho-(1-phenylvinyl)benzoates versatile glycosyl donors for highly efficient synthesis of both O-glycosides and nucleosides | Nature Communications
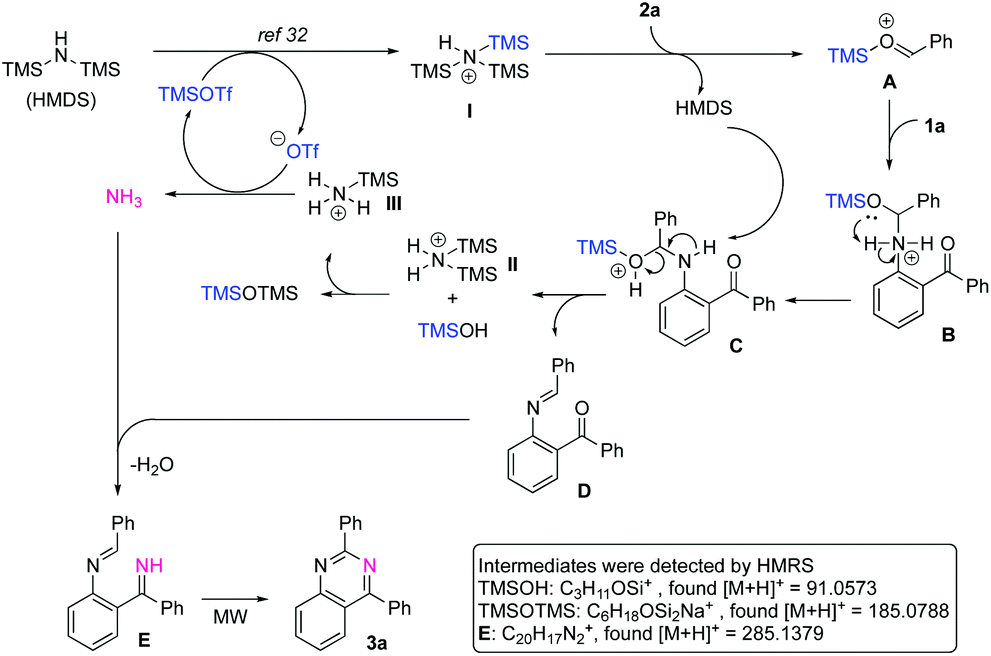
TMSOTf-catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB01507E

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram
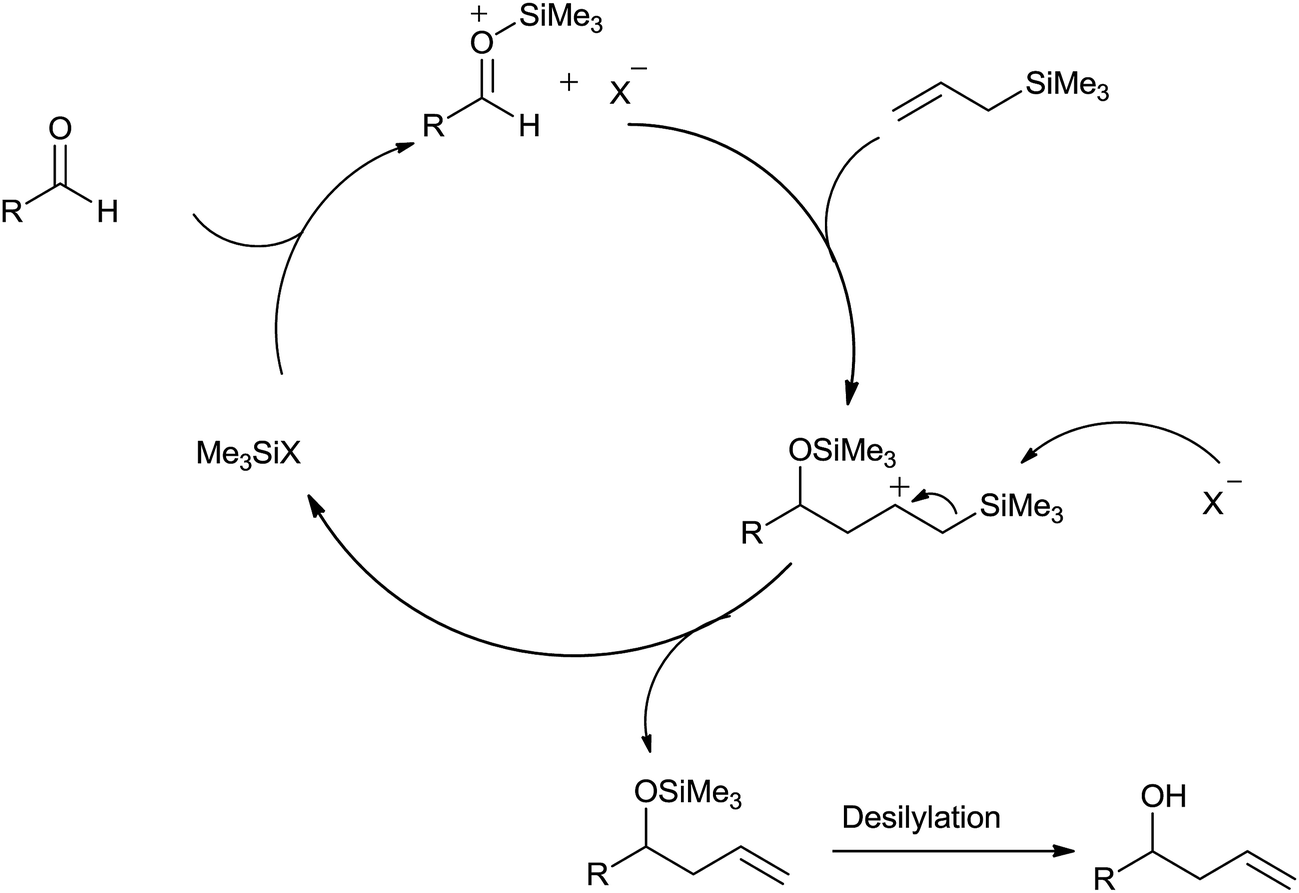
The remarkable journey of catalysts from stoichiometric to catalytic quantity for allyltrimethylsilane inspired allylation of acetals, ketals, aldehyd ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA27813B

The reaction of acetal-type protective groups in combination with TMSOTf and 2,2′-bipyridyl; mild and chemoselective deprotection and direct conversion to other protective groups - ScienceDirect
![Silicon Lewis Acid Catalyzed [3+2] Cycloaddition Reactions of Hydrazones/Cyclopentadiene: Mild Access to Pyrazolidine Derivatives - Zamfir - 2011 - European Journal of Organic Chemistry - Wiley Online Library Silicon Lewis Acid Catalyzed [3+2] Cycloaddition Reactions of Hydrazones/Cyclopentadiene: Mild Access to Pyrazolidine Derivatives - Zamfir - 2011 - European Journal of Organic Chemistry - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/6769206d-9853-43ac-a560-91109269219c/mfig000.jpg)
Silicon Lewis Acid Catalyzed [3+2] Cycloaddition Reactions of Hydrazones/Cyclopentadiene: Mild Access to Pyrazolidine Derivatives - Zamfir - 2011 - European Journal of Organic Chemistry - Wiley Online Library
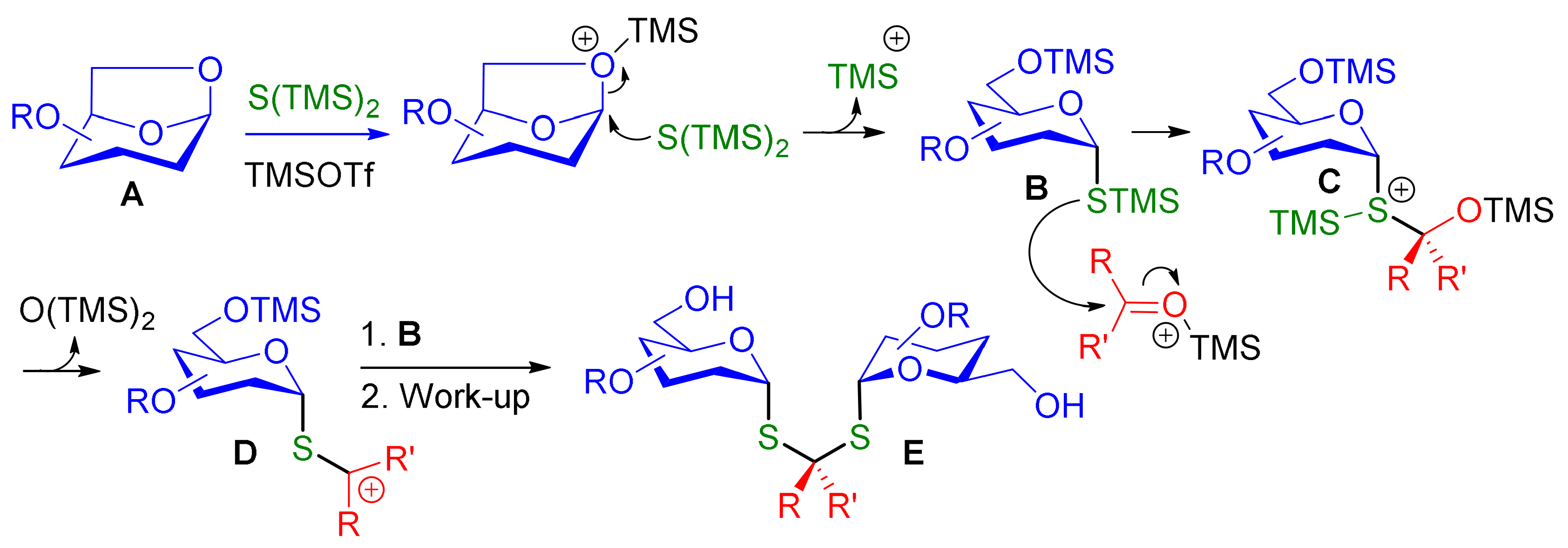
Molecules | Free Full-Text | One-Pot, Highly Stereoselective Synthesis of Dithioacetal-α,α-Diglycosides | HTML
Scheme 3 Proposed mechanism. Reagents and conditions: (i): 3 or 4 with... | Download Scientific Diagram

Scheme 3. Total synthesis of mycalisine B. Reagents and conditions: (a)... | Download Scientific Diagram

O‐Trifluoromethylation of N,N‐Disubstituted Hydroxylamines with Hypervalent Iodine Reagents - Matoušek - 2014 - European Journal of Organic Chemistry - Wiley Online Library

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram
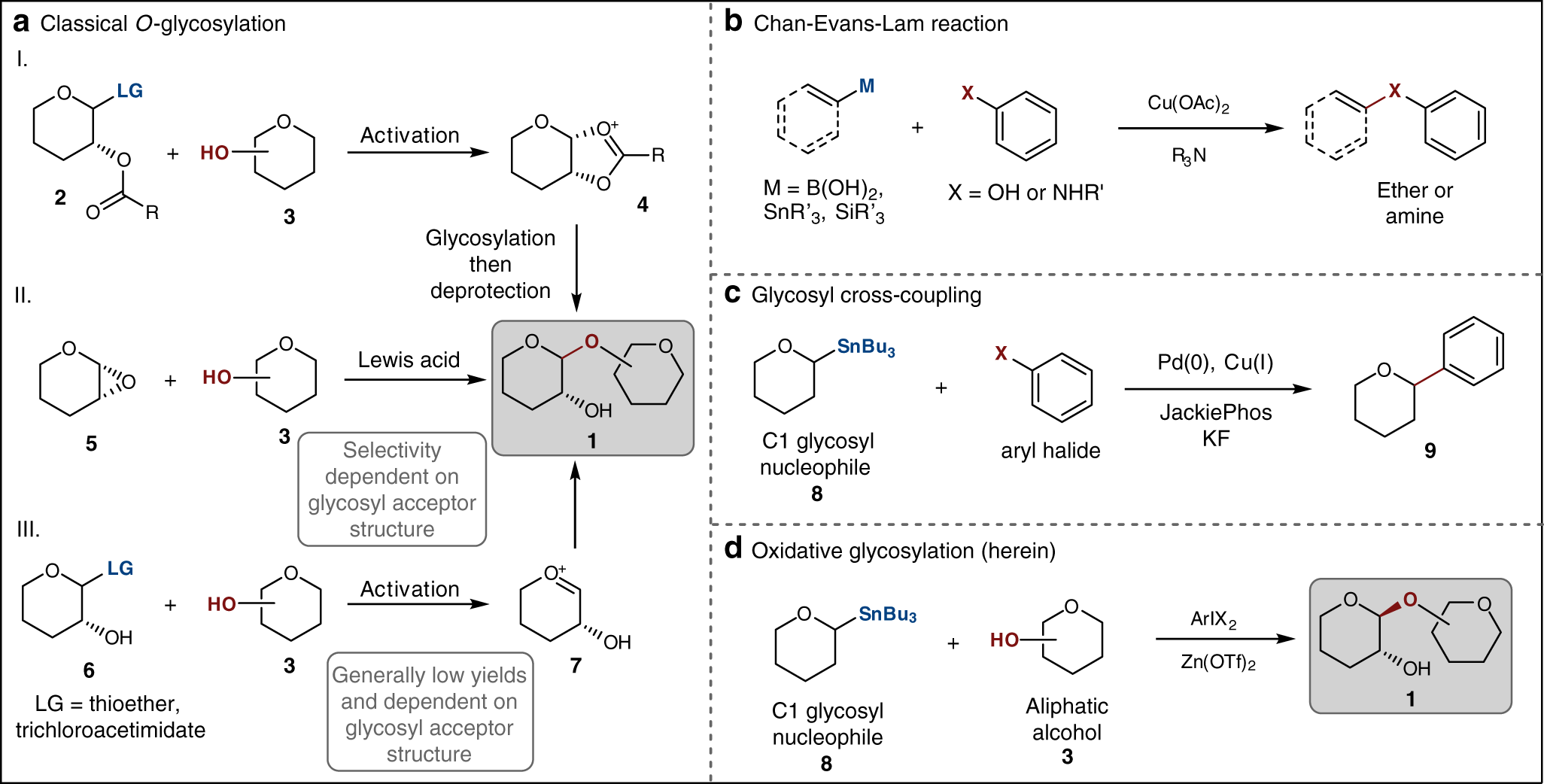
Stereoselective oxidative glycosylation of anomeric nucleophiles with alcohols and carboxylic acids | Nature Communications

Efficient activation of thioglycosides with N-(p-methylphenylthio)-ε-caprolactam-TMSOTf - ScienceDirect
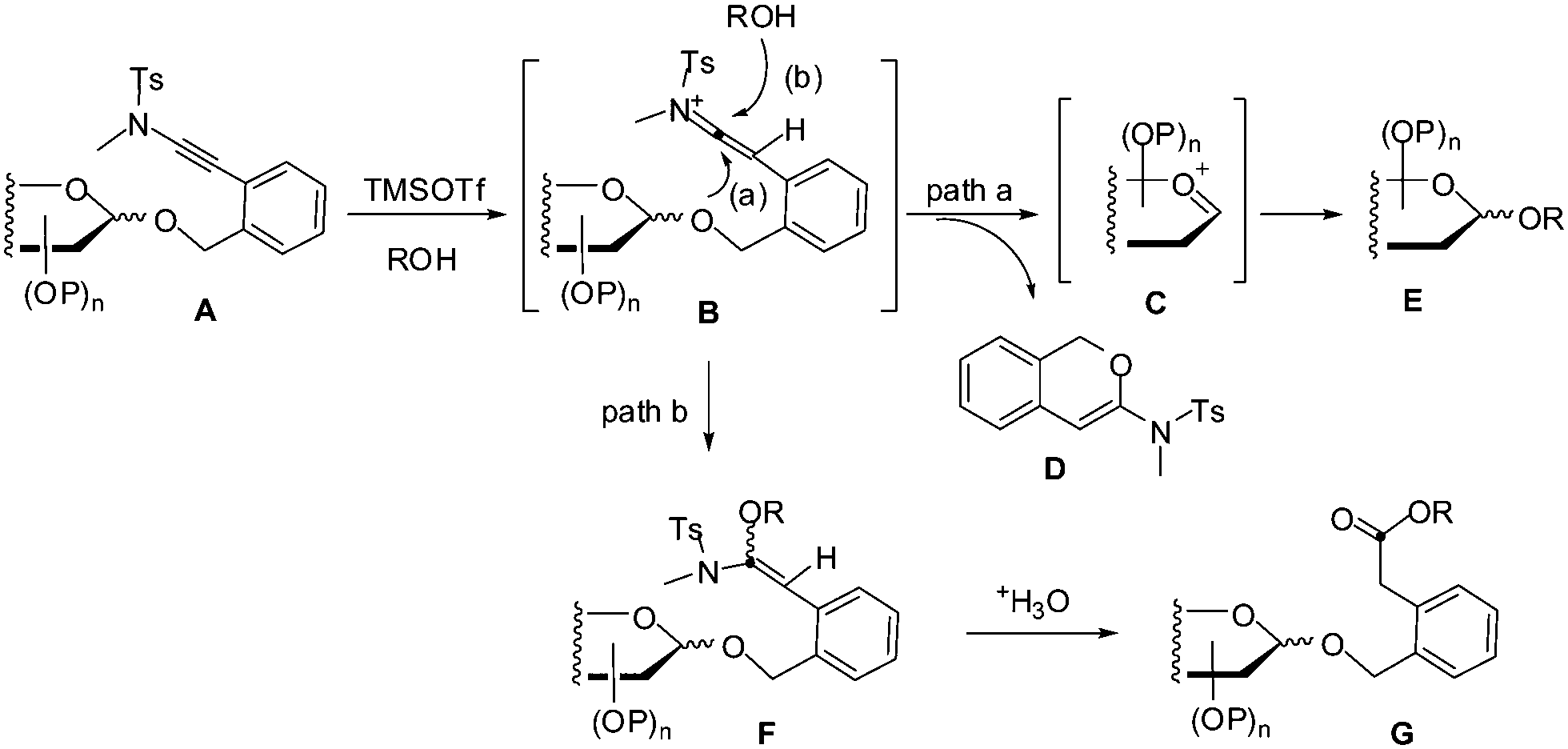
ortho -(Methyltosylaminoethynyl)benzyl glycosides as new glycosyl donors for latent-active glycosylation - Chemical Communications (RSC Publishing) DOI:10.1039/C5CC05651A

Figure 1 from Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3. | Semantic Scholar

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram

N‐Trifluoromethylthiosaccharin/TMSOTf: A New Mild Promoter System for Thioglycoside Activation - Carthy - 2019 - European Journal of Organic Chemistry - Wiley Online Library