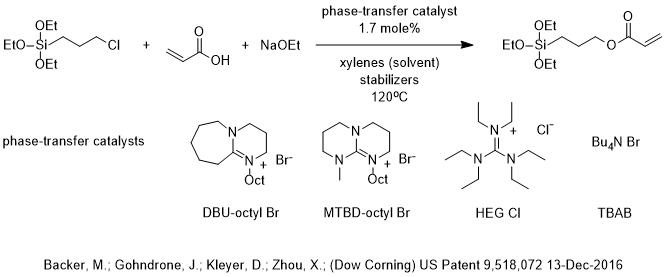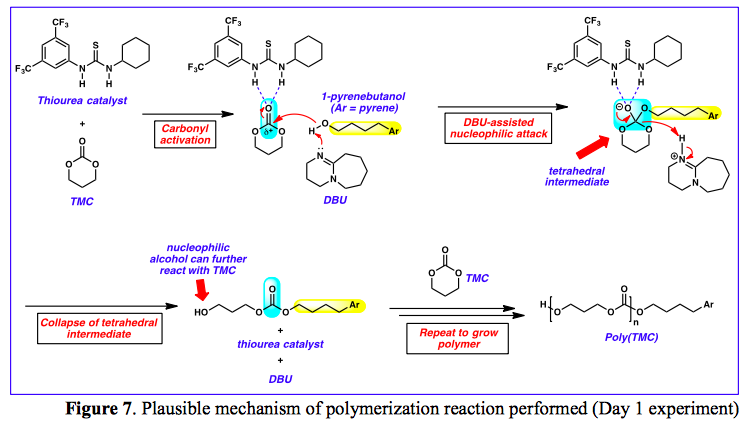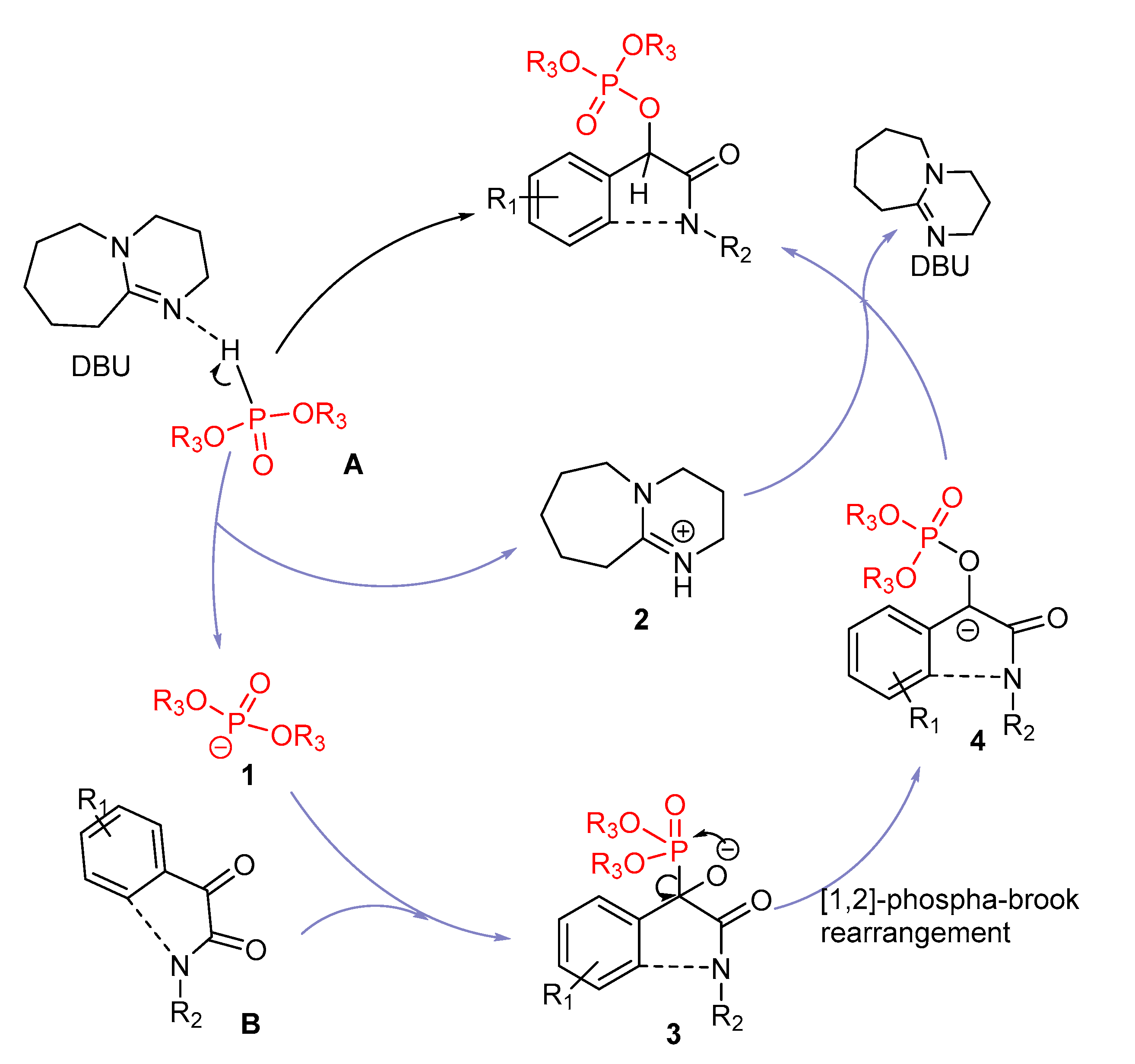
Catalysts | Free Full-Text | DBU Catalyzed Phospho-Aldol-Brook Rearrangement for Rapid Preparation of α-Phosphates Amide in Solvent-Free Conditions | HTML
![Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure - Green Chemistry (RSC Publishing) Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure - Green Chemistry (RSC Publishing)](https://pubs.rsc.org/image/article/2016/gc/c5gc02761f/c5gc02761f-s3_hi-res.gif)
Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure - Green Chemistry (RSC Publishing)
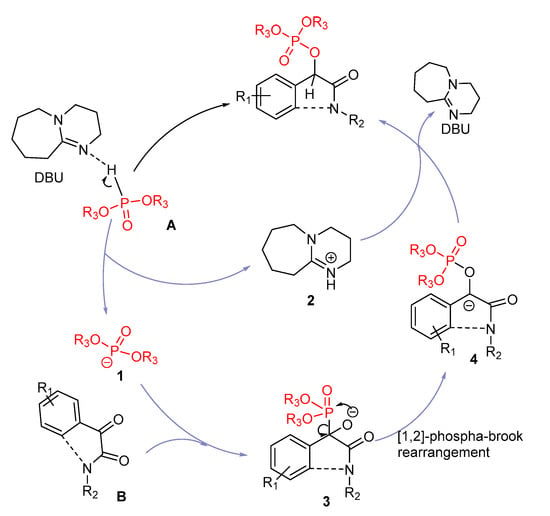
Catalysts | Free Full-Text | DBU Catalyzed Phospho-Aldol-Brook Rearrangement for Rapid Preparation of α-Phosphates Amide in Solvent-Free Conditions | HTML
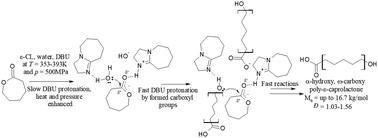
Studying the catalytic activity of DBU and TBD upon water-initiated ROP of ε-caprolactone under different thermodynamic conditions - Polymer Chemistry (RSC Publishing)
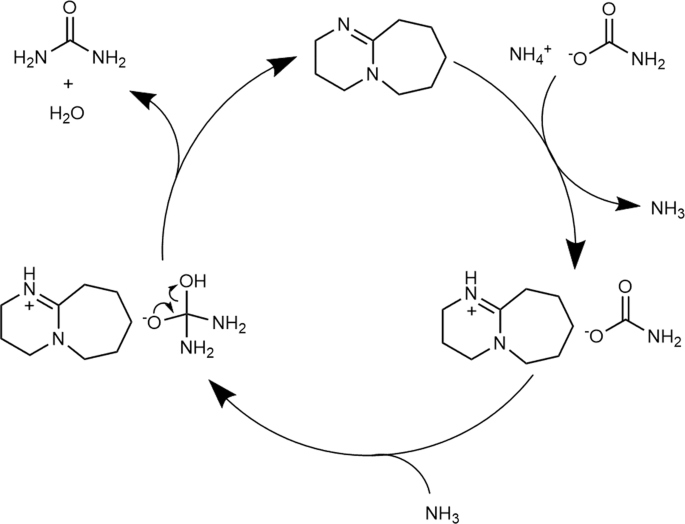
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports

Facile synthesis of DBU-based ionic liquids cooperated with ZnI2 as catalysts for efficient cycloaddition of CO2 to epoxides under mild and solvent-free conditions - ScienceDirect
Organic Catalysis for the Ring-Opening Graft Polymerization of p-Dioxanone with Xylan in Ionic Liquid
![Distinct Promotive Effects of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) on Polymer Supports in Copper‐Catalyzed Hydrogenation of C=O Bonds,ChemCatChem - X-MOL Distinct Promotive Effects of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) on Polymer Supports in Copper‐Catalyzed Hydrogenation of C=O Bonds,ChemCatChem - X-MOL](https://xpic.x-mol.com/20171130%2F10.1002_cctc.201701316.jpg)
Distinct Promotive Effects of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) on Polymer Supports in Copper‐Catalyzed Hydrogenation of C=O Bonds,ChemCatChem - X-MOL
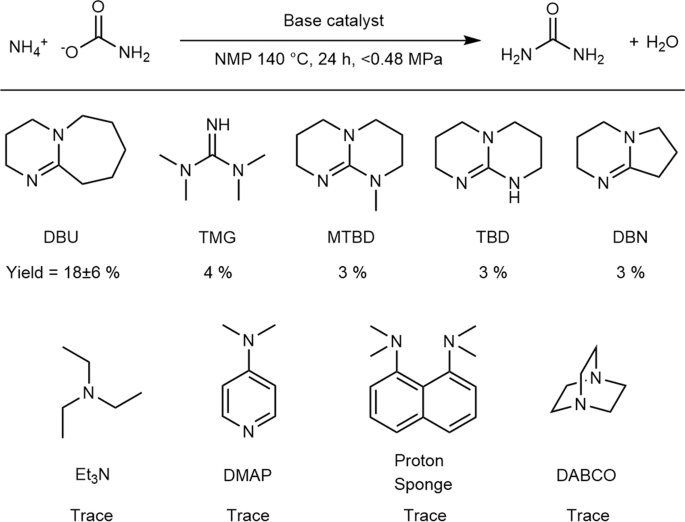
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports
![Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences](https://royalsocietypublishing.org/cms/asset/9fd0eba5-8d4d-40c2-ac1e-45df884f7fbb/rspa20190238f02.gif)
Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalysed by DBU in aqueous ethanol | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences

Unprecedented cooperative DBU-CuCl2 catalysis for the incorporation of carbon dioxide into homopropargylic amines leading to 6-methylene-1,3-oxazin-2-ones - ScienceDirect
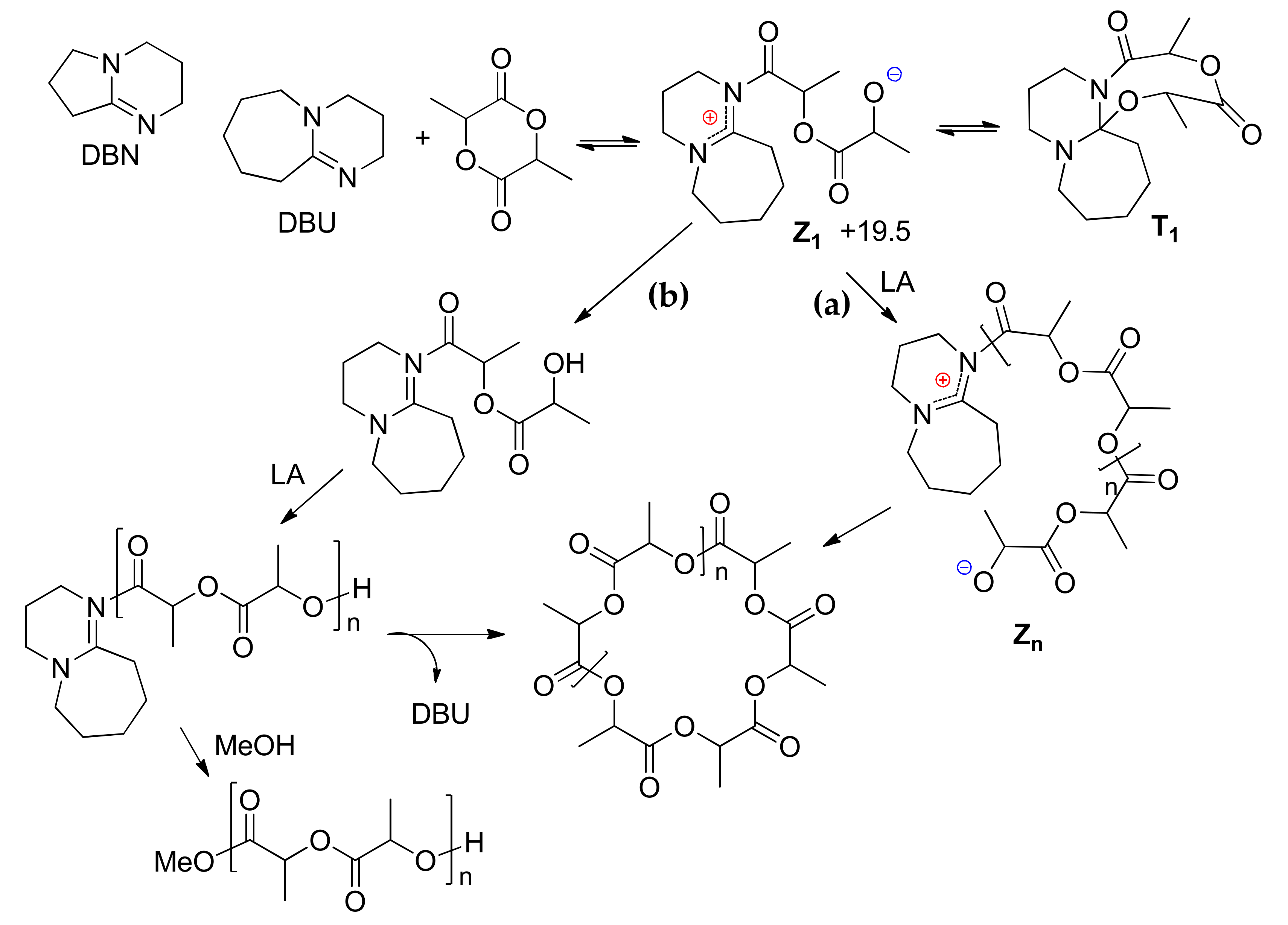
Polymers | Free Full-Text | DFT Modeling of Organocatalytic Ring-Opening Polymerization of Cyclic Esters: A Crucial Role of Proton Exchange and Hydrogen Bonding | HTML
![Scheme 7. Proposed mechanism for the 1,8-diazabicyclo[5.4.0]undec-7-ene... | Download Scientific Diagram Scheme 7. Proposed mechanism for the 1,8-diazabicyclo[5.4.0]undec-7-ene... | Download Scientific Diagram](https://www.researchgate.net/publication/338016321/figure/fig27/AS:846575794393088@1578850806233/Scheme-7-Proposed-mechanism-for-the-1-8-diazabicyclo540undec-7-ene-DBU-mediated.png)
Scheme 7. Proposed mechanism for the 1,8-diazabicyclo[5.4.0]undec-7-ene... | Download Scientific Diagram
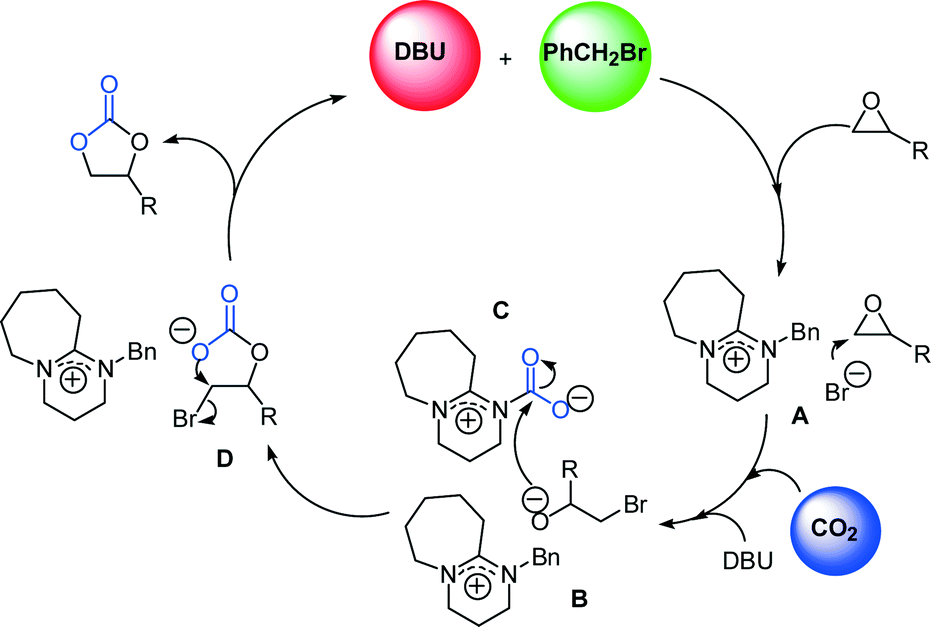
DBU/benzyl bromide: an efficient catalytic system for the chemical fixation of CO2 into cyclic carbonates under metal- and solvent-free conditions - Catalysis Science & Technology (RSC Publishing)

Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry and DFT Studies of Catalyst Role
![Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU). - Abstract - Europe PMC Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU). - Abstract - Europe PMC](https://europepmc.org/articles/PMC5600704/bin/nihms902573f1.jpg)
Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU). - Abstract - Europe PMC
![Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - Organic Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - Organic](https://pubs.rsc.org/image/article/2019/qo/c9qo00602h/c9qo00602h-s1_hi-res.gif)